Chapter 1.1 – Atoms are the Smallest Form of Elements
Historic diary, reflection, or letter from a scientist.
Dear Diary,
Today has been exciting and tired due to the fact that the chemists and I have made some progress my discovering important facts. Apparently 2400 years ago, Greek philosophers believed that earth, water, fire, and air were the four basic substance matter was made up of. But years later, other chemists have proven that information wrong by discovering 100 of different types of elements. They’re the ones that have come to a conclusion that all matter is made up of atoms.
After the discovery of a brief history, I began focusing on the atomic structure of Carbon, and even sketched the atomic structure. Scientist and I have realized that all elements contain protons, neutrons, nucleus, and electrons in their atom. For example, Carbon contains six protons, six neutrons, and six electrons. Protons are positive charged particles as neutrons are uncharged particles. The nucleus, located in the center of an atom, is the combination of protons and neutrons together. The electrons, which are located on the outer of the nucleus also known as electron clouds, are negative charged particles.
I have also realized that all elements contain different atomic masses and different atomic numbers. For example, Carbon’s atomic number is six and its atomic mass is 12.011. An atomic number determines the number of protons an atom contains in an element. An atomic mass is the total number of protons and neutrons a nucleus contains in an atom on an element.
One of the chemists I had been working with had informed me that isotopes exist. Isotopes are atoms of the same elements that contain the same number of protons but different numbers of nucleus. For instance Chlorine – 35 contains seventeen protons and eighteen neutrons. But Chlorine – 37 contains seventeen protons and twenty neutrons.
He had also informed me that atoms can form ions. But how? Elements on the periodic table contain either a positive or a negative charge. An ion can only be formed when an electron of an atom is donated to another atom, or when an atom accepts an electron from another atom.
Anyways, I have a feeling a new discovery is going to be found very soon. Back to work I go!
– Glenn T. Seaborg
Chapter 1.2 – Elements Make up the Periodic Table
Periodical journal article
Dear Diary,
I cannot believe that I have finally completed arranging the periodic table of the elements. It all started in the 1800s when scientists came up with the idea to make a chart of elements and arrange them based on similar properties. I became curious of the elements and wondered exactly how these hundreds of different elements were to be arranged. All I knew was that the elements were to be arranged by similar properties.
First thing I did was make a card for each element that contained its atomic mass and information’s about its properties. The elements were arranged in rows due to similar chemical properties. And then I arranged the elements into rows so that the atomic masses increased as one moved down each column. Finally, the periodic table was completed, and the elements were arranged perfectly due to similar chemical properties, atomic mass, and atomic numbers. I arranged the elements on the period table in such a way that I left empty spaces. I believe that after the periodic table is published, other elements will be discovered and would fill in the chart completely. Luckily I was right. Few years later Aluminum was discovered.
The difference between the first published periodic table and the modern periodic table is, the elements with similar properties are now found in columns, and the elements are also arranged by atomic number.
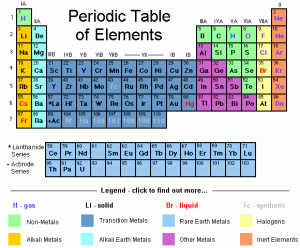
The periodic table is the map of the hundreds of different elements. Periods are read from left to right as groups are read from top to bottom. Periods and groups are useful when you are trying to locate elements on the table. For example, to find Vanadium, go to group 4 and period 5.
I guess that’s it for now. I shall write again as soon as another discovery is found.
– Dmitri Mendeleev
Chapter 1.3 – The Periodic Table is a Map of the Elements
Pictures with reports or thoughts
The periodic table is designed like a map for the hundreds of different elements. The periodic table contains nine different regions: Alkali metals, Alkaline Earth metals, Transition metals, Metalloids, Halogens, Noble gases, Actinides, Lanthanides, and Nonmetals.
Alkali metals, located in group one, are very reactive elements, are reactive with oxygen and with water vapor. These elements are important in life and bond easily with elements in group seventeen.
Alkaline Earth metals, located in group two, are less reactive than alkali metals. They can be found in our diet and body.
Transition metals, located in group three to twelve, are less reactive than most metals. These elements are used for jewelries and can be found in foods as well. They can also be found in money industries.
Nonmetals, the orange elements on the periodic table, are mostly gasses at room temperate except for Bromine. Nonmetals are poor conductors of heat and electric current.
Metalloids, located as the brown elements on the periodic table, have properties of both metals and nonmetals. They make up semiconductors and are found in electronic devices.
Halogens, located in group 17, are very reactive nonmetals that are often used to kill microorganisms. They also form salts with metal, such as potassium chemical salt.
Noble gases, located in group 18, are almost never reactive with other gases.
Lanthanides, located on the top row of the two rows at the bottom of the periodic table, are very hard to locate in pure form and are hard to isolate.
Actinides, located on the bottom row of the two rows on the periodic table, are very radioactive and can be uses of radioactivity to kill cancerous tissue.
Chapter 2.1 – Elements Combine to Form Compounds
Newspaper Article
THE BERYLLIUM TIMES ARTICLE
Thousands and thousands of years ago, scientists have discovered that all matter are made up of atoms. All matter is also made up of different types of compounds. A compound is a combination of one or more different elements. For example: when two Hydrogen atoms and one Oxygen atom bond, they create a water molecule. All compounds contain chemical and physical properties. Water becomes solid at 0 degrees Celsius, and evaporates at 100 degrees Celsius. What are the physical properties when water reaches to 0 degrees Celsius? It becomes hard and solid. And what happens when water reaches to 100 degrees Celsius? Water evaporates and eventually becomes a gas. And there, we have a change in physical properties.
But what happens when calcium, a soft, silvery metallic solid, and chlorine, a poisonous gas, combine? When calcium and chlorine combine, they create Calcium chloride which is a white solid, useful for melting ice that form on streets in the winter season. And there, we have a change in chemical properties.
A chemical formula regards how many atoms are contained in a compound by using symbols of the elements. For instance, Ammonia contains one nitrogen atom and three hydrogen atom. In result the chemical formula for ammonia would be: NH3
Scientists use chemical formulas to show what happens after atoms of elements combine.
For example: 2H + O => H2O

Article written by Afifa Imam.
Chapter 2.2 – Chemical Bons Hold Compounds Together
Historic diary, reflection, or letter from a scientist
Dear Glenn Seaborg,
Remember the explanation I gave you about atoms forming ions? Well I have found more important information to add to the explanation. When atoms form ions, there is a bond between the two atoms. In fact there are two different types of bonds. These two different bonds between electrons are known as ionic and covalent bonds. Ionic bonds accept and donate electrons from atoms of elements, as covalent bonds share electrons from atoms of the elements.
I have made a copy of the periodic table and wrote on top of columns the charges of the elements. Group 18 does not have any charges as for all the elements under group eighteen contain eight electrons on the valence electrons. No electrons are needed to make a perfect eight on the valence electron.
Example of an Ionic Bond:
What happens when a hydrogen atom and a fluorine atom bond?
A hydrogen atom contains one electron on the valence electron, as for a fluorine atom needs one more electron to complete a perfect eight on the valence electron. So when a hydrogen atom and a fluorine atom bond, hydrogen’s only one electron donates it to the fluorine atom. When fluorine accepts the electron from hydrogen – the two atoms bond together and create Hydrogen fluoride .

Example of a Covalent Bond:
An oxygen gas is an example of a covalent bond. An oxygen atom contains six electrons in the valence electron. This means that two electrons are needed to complete an eight in the valence electrons of oxygen. So what happens when two oxygen atoms bond? An oxygen atom shares two of its electrons to the other oxygen atom. When two oxygen atoms bond they become an atom of an oxygen gas.
Write me back when you have found another discovery. Take care for now
– Barnard Courtois (discovered Iodine, 1811)
Chapter 2.3 – Substances’ Properties Depend on their Bonds
Diagram of a process with technical writing inserted

Chapter 3.1 – Chemical Reaction Alter Arrangements of Atoms
Newspaper Article
THE EUROPIUM TIMES ARTICLE
An optimistic scientist, Albert Smith, has been experimenting with substance for many years now. Albert Smith has been trying to find evidences of chemical reactions, types of chemical reactions, and things that affect the rates of a chemical reaction. During his experiment, Smith has realized the difference between both physical and chemical changes, and also discovered reactants and products in chemical formulas.
What are chemical and physical changes? A physical change is a change in the state after a chemical reaction. For instance, when solid water melts and becomes a liquid, the water molecules re-arrange but the molecules itself remain the same after a change in state.
What are chemical changes? A chemical change is when a substance changes into another substance. For instance, when the substance vinegar and baking soda combine, a formation of gas and a fizzing noise occurs.
In a chemical reaction, a change in color may occur. For example when gray iron rusts, it becomes brown. Also there may be a formation in precipitate. When two liquids react a solid product also known as precipitate, may form. When sea creatures release liquid – the sea water and the liquid may react forming a type of a solid product. A formation of a gas and a change in temperature may also occur in a chemical reaction.
A chemical reaction can be classified in two ways: Synthesis, Decomposition, and Combustion. A synthesis reaction is the combination of two or more reactants. The chemical formula 2H + O => H2O is defined as a synthesis reaction. Decomposition is when reactants are broken down into simpler products. The chemical formula H2O => 2H + O would be classified as a decomposition reaction. We all may be wondering what reactants and products are. The substances presented before a chemical reaction is known as the reactants, and the substances formed by a chemical reaction is known as the product.
Last but not least, Smith has realized that a change in temperature, concentration, increase in surface area, catalysts and stirring, may all affect the rate of a chemical reaction.
- In a change in temperature, the rate of a reaction can be increased by making particles move faster.
- A high concentration of reactants means that there are large numbers of particles that can collide and react.
- Increase in the surface area of the reactants increases the number of particles that can interact.
- Stirring causes particles to move faster together as well as a reaction occurs faster. Adding energy.
- Catalyst is a substance that increases the rate of a chemical reaction but itself does not change after a complete chemical reaction.
Keep reading the Europium times article to keep updated with new discoveries.
Article written by Afifa Imam
Chapter 3.2 – The Masses of Reactants and Products are Equal
Dialogue between two people
[ The Adventures of Spongebob and Patrick ]
S: Hey Patrick.
P: Yeah Spongebob?
S: I need help with my home-work.
P: Sure what is it?
S: What does the law of conservation state?
P: The law of conservation states, “In a chemical reaction atoms are neither destroyed nor created”.
S: Oh okay, thank you.
P: Yo Spongebob?
S: Yes Patrick?
P: How do you balance chemical equations like __N2 + __H2 = __ NH3
S: Well the point of this chemical equation is to make both sides of the equation equal.
P: I know…
S: So we know that on the left side of the equation there are two nitrogen atoms and two hydrogen atoms. And there are one nitrogen atom and three hydrogen atom combined on the right side.
P: yeah…
S: So in order to make both sides of the equation equal, we add three NH3 on the right side of the equation and one N2 and three H2 on the left side of the equation.
1 N2 + 3 H2 = 2 NH3
P: okay…
S: so now there are two nitrogen atoms on both side of the equation and six hydrogen atoms on both sides of the equation. And there, both sides of the equation are equal!
P: Oh okay, I get it now. Thanks Spongebob.
P: (turns to Mr. Krabs) I don’t get it at all!
Chapter 3.3 – Chemical Reactions Involve Energy Changes
Dialogue between two people
[ The Adventures of Spongebob and Patrick, Part II ]
P: *whispers* Psssssttt Spongebob!
S: Patrick I’m busy.
P: Just for a minute. Please!
S: Okay fine? What?
P: What is an endothermic reaction?
S: An endothermic reaction is a reaction when energy is absorbed or needed.
P: Like what?
S: For example, plants absorb heat from sunlight to turn carbon dioxide and water into oxygen and glucose.
P: Okay then what’s an exothermic reaction?
S: Exothermic reaction is a reaction when energy is being released.
P: Yeah? Like what?
S: For example, glow sticks work by a chemical reaction that releases energy as light.
P: Oh okay…
S: Why?
P: I just felt like asking…
S: Well did you understand what I explained to you?
P: No, not really.
S: God Patrick! I’m busy!
Chapter 3.4 – Life and Industry Depend on Chemical Reactions
Diagram of a process with technical writing inserted
Chapter 4.1 – A Solution is a Type of Mixture
A short story (other writing techniques)
The Adventures of Billy and Bob
One hot sunny day, Billy and Bob decided to make solution of Kool-aid powder and cold water together. A solution is a mixture of the solute and the solvent together. In this situation the solute is considered as the Kool-aid powder, and the solvent is considered as the cold water.
We also know that this solution is not considered as a suspension as for if it was its appearance would look like a liquid cloudy. A suspension is when a particle is larger than other particles in the solution.
After making a solution of Kool-aid and cold water – Billy and Bob came up with the idea of pouring it into the ice cube container and leaving it in the freezer so that it would become Kool-aid ice cubes. But Billy and Bob were both very impatient and couldn’t wait.
“My teacher told us in class recently that when more solutes are added to a solution, it decreases the freezing point of the solvent,” Billy informed.
Bob thought for a while and then asked, “Oh and when more solutes are added to a solution, it increases the boiling point of the solvent… right?”
“Right, but who would want to boil Kool-aid?”
“If it’s too cold to drink Kool-aid in winter; you could always boil it…”
“That’s a smart idea!”
The Kool-aid ice cubes were finally frozen, and the two boys enjoyed biting and licking their frozen solution.
Chapter 4.2 – The Amount of Solute that Dissolves Can Vary
A short story (other writing techniques)
The Adventures of Billy and Bob (Part II)
One cold winter day, Billy and Bob came back home from playing basketball out at the park.
“I’m freezing cold, let’s make hot chocolate,” Bob stuttered.
The two boys ran to the kitchen as fast as they could. First they got two cups out and put two teaspoons of chocolate powders, and one cubes of sugar in both cups. Then they poured hot water. Billy was the first one to taste the hot chocolate drink.
“This hot chocolate is not sweet enough,” Billy complained.
Both Billy and Bob increased the concentration of both drinks by adding two cubes of sugar. A solution’s concentration is the amount of solute that can dissolve in a solvent at a given temperature.
After adding two cubes of sugar, Bob tried tasting the hot chocolate this time and thought it tasted too sweet. The boys diluted the hot chocolate drink by increasing the amount of solvent, also known as the hot water. The hot chocolate was no longer sweet or plain. A solution that contains large amount of solvent is called a dilute.
After Billy and Bob finished their drink, they decided to get a huge jar and fill it up with hot water and chocolate powder.
“Hey Bob, let’s saturate this solution!” Billy exclaimed.
The boys added the maximum amount of chocolate powder it can dissolve into the jar of hot chocolate. Billy and bob watched the chocolate powder dissolve in the hot water. This solution is saturated because the solution contains the maximum amount of solute it can dissolve in a solvent at a given temperature.
After a while, Billy poured half glass of hot water into the jar. The hot chocolate is now unsaturated because it contains less than the maximum amount of solute that can be dissolved in a solvent at a given temperature.
“Dude let’s supersaturate the hot chocolate!” Bob suggested.
“What do you mean?” Billy asked.
“Well since we have already saturated the hot chocolate, we can still increase the amount of solute than its maximum amount by boiling it.”
“Okay fine, let’s try it.”
So then Billy and Bob took the pan out and poured the jar of hot chocolate into it and placed it on top of the fire. After a minute of two, Bob continued to add more chocolate powder while Billy stirred.
Both Billy and Bob knew that the drink was going to be so bitter that neither of them wanted to taste it for themselves.
When the two boys heard their mother walk into the living room, they stopped the fire and ran out of the kitchen and into the living room.
“Hey boys, how was school?”
“It was fine,” Billy answered
“Did you learn anything interesting today?” their mother asked.
“Well… we learnt that temperature and pressure effects solubility.”
“What exactly is solubility?” their mother tested them.
“Solubility is the amount of substance that will dissolve in an amount of solvent at a given temperature.” Bob explained.
“Fair enough, I was just checking if you boys really paid attention.”
Chapter 4.3 – Solution Can be Acidic, Basic, or Neutral
Periodical journal article
Afifa Imam’s Diary
Dear Diary,
While I was in science class, which is one of my favorite classes of the day, I have happened to take good notes in class to help me study for the up coming quiz that I know we’re going to have very soon. At the moment we are learning about acids, bases, and their characteristics and properties. We are also learning about the pH scale and neutralizing acids and bases.

A pH scale is a scale that is used to measure the concentration of hydrogen ions in a solution. The numbers on the pH scale are measured from 0-14 where zero means there are high concentration of hydrogen ions, and fourteen means there are low concentration of hydrogen ions. Seven means the solution is neutral. Water for example is a neutral solution. It is neither acidic nor basic. On the pH scale a high concentration of hydrogen ions are usually indicated by a low number and a low concentration of hydrogen ions are usually indicated by a high number.
Chapter 4.4 – Metal Alloys
Pictures with Reports or Thoughts
What are alloys? An alloy is a substance that is a mixture of a metal and one or more different types of elements. Solids and gases can be considered as solutions. Alloys, which is a solid solution, is usually made by melting and then cooling the solution. For instance bronze is made by melting both copper and tin. In this solution tin is the solute and copper is the solvent. After the solute and the solvent mix; the solution is to be cooled down so that the solution becomes a solid. Most alloys are useful in transportations, medicines, and in space flights. They are also useful in jewelries and most accessories.
Brass are useful in instruments, faucts, and jewelries.
Bronze are useful in hardware for boats, screws, and grillwork.
Stainless Steel are useful for tableware, cookware, and surgical instruments.
Carbon steel are useful for tools, machinery, and rails.
Pewters are useful for making sculptures.
Chapter 5 – Carbon Chemistry
Persuasive advertisement that educates